Light It Up!
As we dive into the annual festive season, we see all sorts of decorative lights hanging in the streets, in the trees and in our homes. But let’s not forget that in our watch boxes, there are also small little machines with their own decorative, and functional, lights. Just exactly how does our watches light up? We have a nerdy write up today to go into the science of illumination. The mechanics of the lume.
Physics of illumination
Be it Christmas LED lights, television or the backlit screens on the classic F91W, the reason we see light is because there are packets of energy, called photons, entering our eyes. And when these photons vibrate at a particular wavelength, we see them as a particular colour. It is probably much easier if we looked at some familiar watches and explain from there.
LED
One of the most efficient form of illumination is the light emitting diode, or LED as most of us know it. A current passes through a semiconductor which causes electrons to move, releasing photons.

A more modern, but still classic example would be the Casio F-91W, which some Singaporeans would call the Army watch. It’s low cost, high versatility and ultra light weight makes it a great choice for intense outdoor training in the military. The LED backlight on this is green, suggesting that indium gallium nitride was used in the semiconductor.

A newer, and higher end and fully mechanical watch that uses LED is the impressive HYT H4 Alinghi. I say impressive not so much because of the LED technology, but the engineering efforts to miniaturize a brushless dynamo into a corner of the watch, eliminating the need for a battery or electrical energy storage.

Electroluminescent
LED bulbs tend to give an uneven illumination since it is a small bulb tucked away at the edge of the dial. Along comes electroluminescent (EL), or commonly known as Indiglo in Timex watches. Casio’s F-105W and uncountable other digital watches uses EL backlight in place LED bulbs for aesthetics reasons and better night time legibility. Underneath the dial, there is a very thin film consisting of essentially 4 layers: a conducting layer, a phosphor layer, an insulator and another conducting layer. When a current is applied across the thickness, the electrical current energizes the phosphor and causes photos to be emitted evenly across the film.

Phosphorescence
Phosphorescence, or lume, is the go-to passive method of illumination on watches. We love our lume. There are even hashtags for lume like #frinightlume #lumeparty. What’s not to love about that gentle glow? To better describe how the glow is created, we’ll have to get into some very technical discussion that’s usually left for physicists. Phosphors are a group of materials that have the ability to absorb light energy (even if slightly outside the visible band, such as UV or IR) to bring electrons to an “excited” state. Once the energy source is removed, the material can continue a slow decay where the electrons will jump back to their “relaxed” stated. During which, the energy is released as photons of specific wavelength. There are a few types of phosphors, but most commonly used is zinc sulfide and strontium aluminate. This slow decay happens over a time frame between minutes to hours depending on the age and quality of the lume.

Iconic blue glow of the Chromalight. Full article on the Deepsea Sea-Dweller here.
A prominent lume that most readers have seen is the chromalight by Rolex, which has a signature blue tint instead of green. We now understand that the glow comes from a photon being released as the electrons jump from “excited” to “relax” states. Going one step deeper in the science, it is also possible to tune the colour we want, by adding in various activators to control the wavelength of the released photons. In the familiar green glow we know, the dopant is copper and releases photons around 535nm. In the more exotic Chromalight, europium is used and the releases photons at wavelength of about 490nm.

Radioactive activation
Most lume only hold their charge for under an hour. Of course good watch brands such as Rolex are able to produce paints with superior phosphorescent properties and even then, it tends to last no more than a few hours. Pretty sure you have noticed that as the paint ages, it holds less charge and shows reduced brightness and glow time. To overcome the short glow time, some other form of activation has to be used. Introducing radioactive emission. As radioactive materials decay, they emit energy that can be absorbed by phosphor, and then the phosphor does it’s magic where electrons that surround it jumps from a higher state to a lower more stable state, and releases a photon in the process. As the radioactive material does not stop decaying, it is constantly bombarding the phosphor with electron beams which creates a persistent glow by the phosphor.

Almost a century ago, radium was the radioactive material of choice to be put in lume paint. On its own, it appears as white. Radium and all its isotopes are highly radioactive and glows on its own without further addition of phosphors. However, the detrimental health effects soon caught attention and it was completely phased out, albeit taking decades to be completely eliminated. In more recent history, radioactive material tritium (3H, a radioactive isotope of Hydrogen) became a more popular choice as shown in the Sinn EZM1. In those days, the tritium loaded paint (and radium) was applied as a paint. While harmless to the wearer, the operators who applied the paint manually were exposed to an amount that accumulated and became harmful. More so for those who had the habit of licking their paint brush tips to get it sharp for these precise applications. Eventually, tritium also became less popular and got replaced by light-activating lume.

In more recent years, engineers worked around the safety aspect of using radioactive materials and introduced the concept of using sealed tubes of tritium gas. Mb-microtec manufactures these tubes with the brand name Trigalight.

It’s a long glass tube internally coated with a phosphor (zinc sulphide with customizable dopants in this case) and then filled with tritium gas to an optimal pressure. The higher the pressure, the more radiation to activate the phosphor and thus increasing brightness. The long glass tube is then laser cut into the small required lengths, with the laser sealing the ends as it cuts. Although it will not need re-charging, the overall life of the lume depends on the half life of the tritium gas (about 12 years).
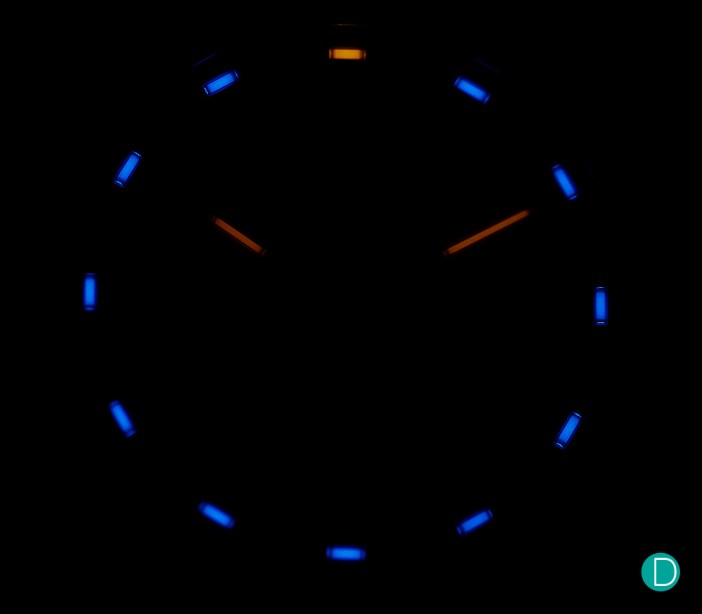
Probably about half the watches available comes with some form of illumination; from the most economic Casio to haute horology such as A. Lange & Söhne Datograph Up/Down Lumen. You’re likely to own at least one that illuminates, so pick out your favourite and go join the festive night lights this holiday season!